Many interesting compounds are liquids or gases at room temperature, and Edinburgh is one of only a handful of chemistry departments in the world where expertise is available for the determination of the crystal structures of these materials.
This type of experiment allows the structures of the very simplest molecules to be determined in the solid state - molecules unencumbered by steric effects of bulky substituents. We can, of course, also solve structural problems that are resistant to other techniques like NMR in the case organic molecules, which exhibit rapid torsional flexibility. For low temperature experiments, the sample is contained within a capillary, which is mounted directly on the diffractometer. The sample is then crystallised in situ by means of a zone refinement technique using an infra-red laser. Data are collected in the usual way.
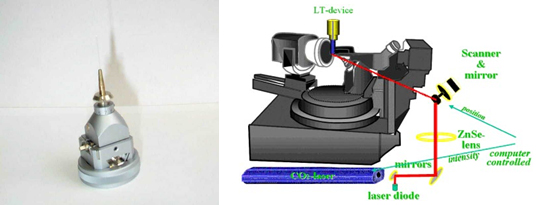
Figure left: Goniometer head with fibre attachment used for single crystal measurements. Right optical heating and crystallisation device
The sample is placed into a glass capillary, which in turn is mounted on a goniometer head. The sample is then placed on the diffractometer and a single crystal is grown in situ with an optical heating and crystallisation device (OHCD). The OHCD device is an infra-red, 25 Watt CO2 laser which is used to heat the sample under study. The laser beam position and power output are computer controlled for accuracy.
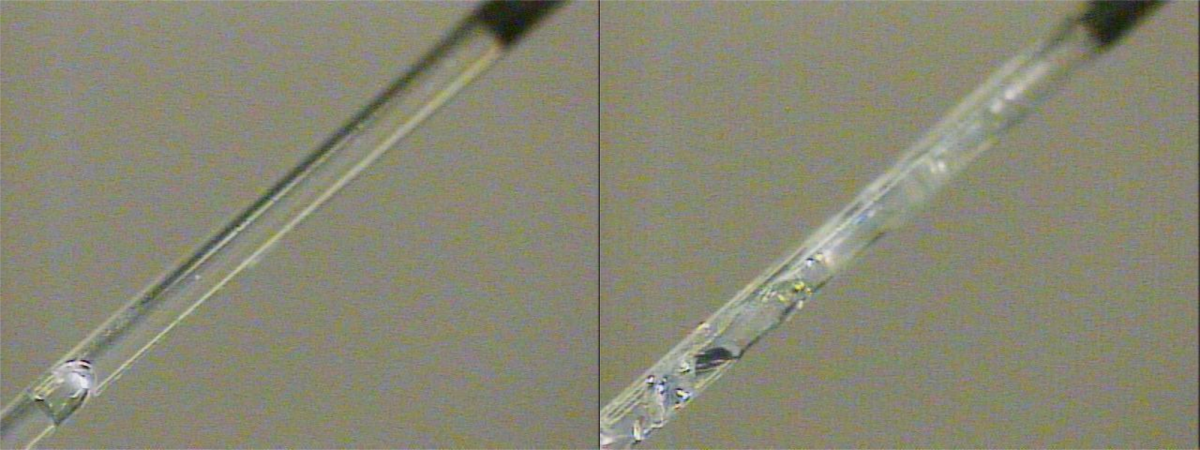
The sample is cooled using the cryostream until solid. However, supercooling can occur where by the sample remains a liquid at a temperature beneath its melting point (left). Flash freezing the sample usually induces glass formation (right).
By moving the laser of the OHCD device along the length of the capillary (left), the glass may be annealed into a polycrystalline powder (middle). This can be observed both from the appearance of the sample and its diffraction pattern (right).

Careful manipulation of the power of the laser, together with the time taken to scan the laser up and down the capillary containing the sample, slowly induces single crystal growth (left). The quality of the crystal is constantly monitored using its diffraction pattern (right). Growth after 6 hours is shown
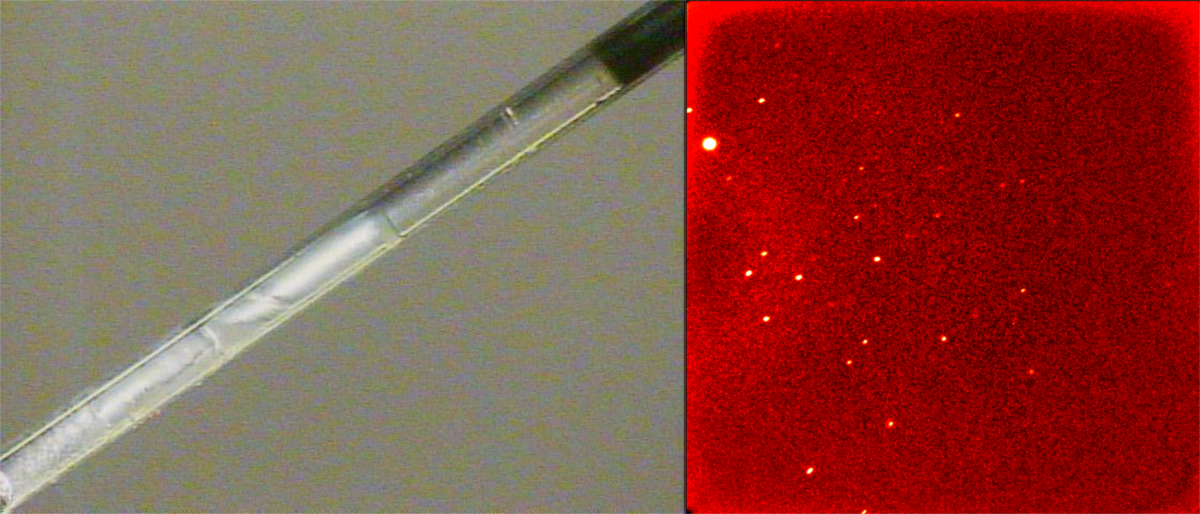
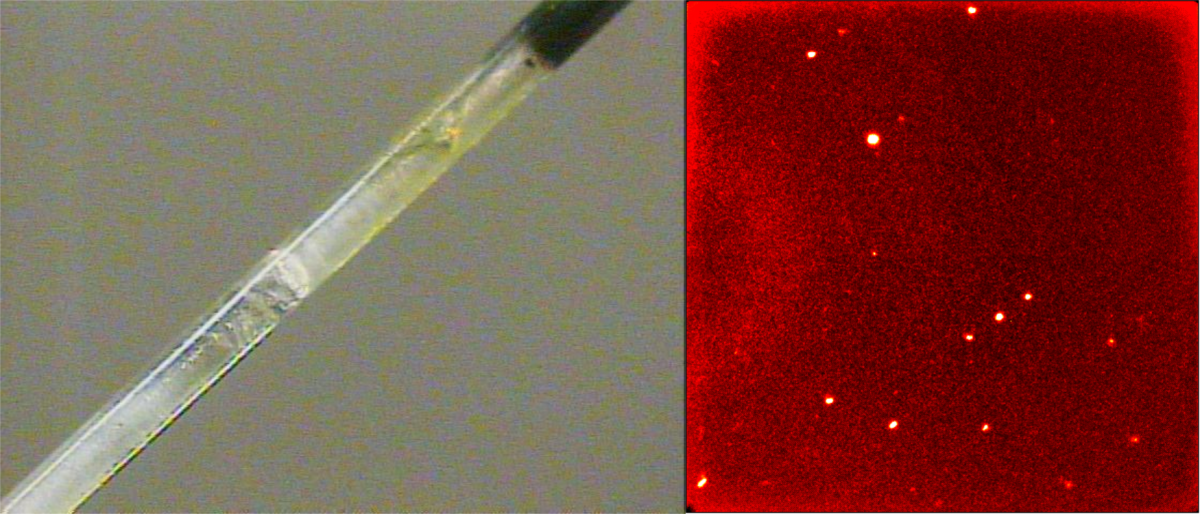
After growing a crystal and zone refining it with the laser for 12 hours the diffraction pattern has improved dramatically and the crystal growth and the correspoding diffraction pattern are shown.
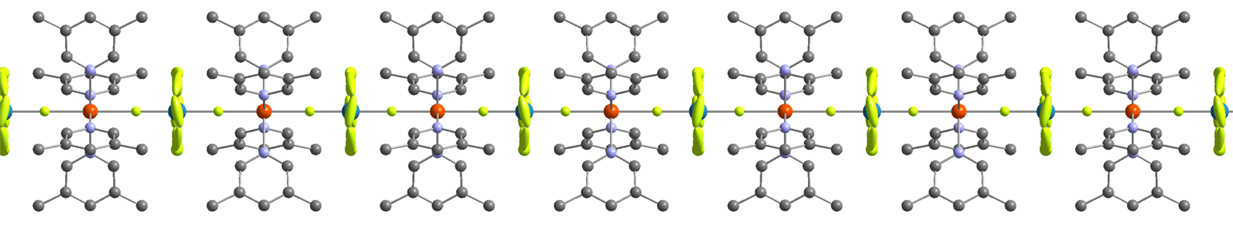
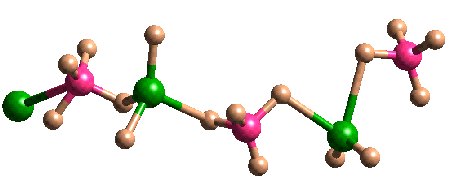
By combining this experimental technique with that of gas phase electron diffraction (see Prof. Rankin), very simple molecules can be studied in the solid and gas phase. A recent example of such a combined investigation is shown in the structure of GaBH6. Unlike the structure in the gas phase, in which GaBH6 forms a similar structure to that of diborane, the GaBH6 molecules forms a helical polymeric structure in the solid state.
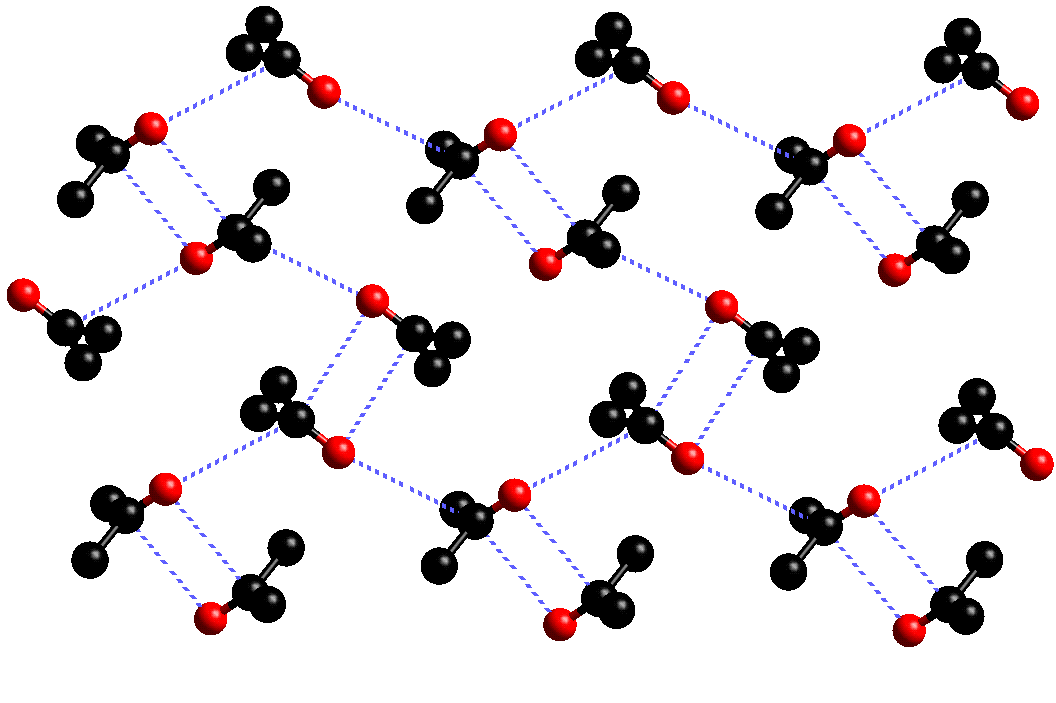
The structure of acetone has been studied at both low temperature and at high pressure. At low temperature, acetone has been shown to crystallise in two phases; a metastable C-centred orthorhombic phase and a stable primitive orthorhombic phase. The metastable phase resembles that of the high pressure structure, having a C=O···C=O distance of 3.587(3) Å, which is significantly greater than that observed in the high pressure studies. The metastable phase readily decomposes to the primitive phase, in which acetone forms two layers (A and B) as shown.
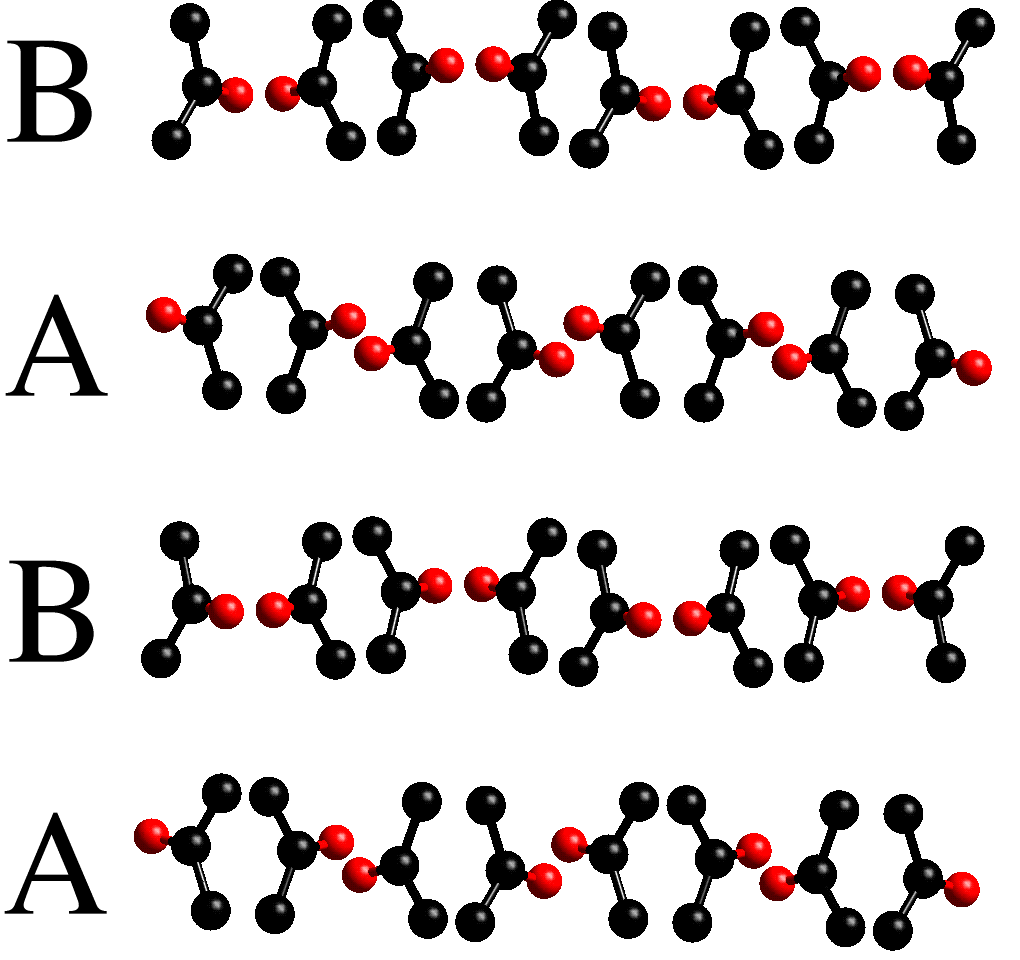
In layer A, the acetone molecules form a "chain" like structure, with head to tail C=O···C=O contacts (3.458(3) Å)
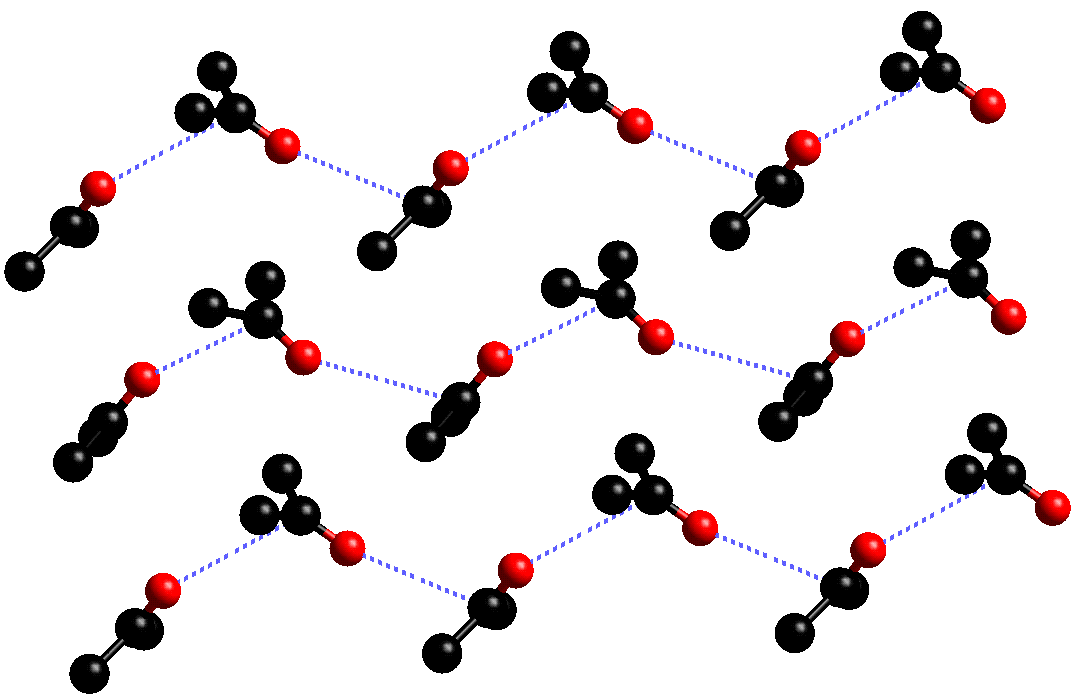
In layer B, each acetone molecule forms close contacts with three neighbours. The C=O···C=O contacts between "pairs" of acetone molecules is observed to be 3.300(3) Å, while those between the "single" acetone and the associated "pair" is 3.458(3) Å.