The Effect of High Pressure on Polymorphs of a Derivative of Blatter’s Radical: Identification of the Structural Signatures of Subtle Phase Transitions., Crystal Growth & Design, 2023, 23(3), pp.1915-1924.
Edward T. Broadhurst, Cameron J. G. Wilson, Georgia A. Zissimou, Mayra A. Padrón Gómez, Daniel M. L. Vasconcelos, Christos P. Constantinides, Panayiotis A. Koutentis, Alejandro P. Ayala, and Simon Parsons.
The effect of pressure on the α and β polymorphs of a derivative of Blatter’s radical, 3-phenyl-1-(pyrid-2-yl)-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl, has been investigated using singlecrystal X-ray diffraction to maximum pressures of 5.76 and 7.42 GPa, respectively. The most compressible crystallographic direction in both structures lies parallel to π-stacking interactions, which semiempirical Pixel calculations indicate are also the strongest interactions present. The mechanism of compression in perpendicular directions is determined by void distributions. Discontinuities in the vibrational frequencies observed in Raman spectra measured between ambient pressure and ∼5.5 GPa show that both polymorphs undergo phase transitions, the α phase at 0.8 GPa and the β phase at 2.1 GPa. The structural signatures of the transitions, which signal the onset of compression of initially more rigid intermolecular contacts, were identified from the trends in the occupied and unoccupied volumes of the unit cell with pressure and in the case of the β phase by deviations from an ideal model of compression defined by Birch−Murnaghan equations of state.


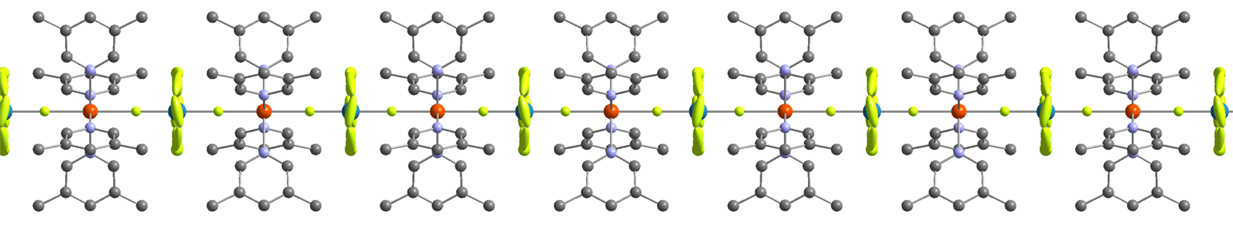
 H contacts at the transition. Although no dimerization of the radicals occurs, the π-stacking interactions are compressed by 0.341 (3) Å between ambient pressure and 6.07 GPa.
H contacts at the transition. Although no dimerization of the radicals occurs, the π-stacking interactions are compressed by 0.341 (3) Å between ambient pressure and 6.07 GPa.